On March 13, the metabolic cardiovascular disease research team of Affialted Union Hospital published the article "Poly (ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2" in Nature Communications. This was the second research achievement of the team published in Nature Communications following the article “Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells” on February 28.
The metabolism disorder of calcium and phosphorus in patients with chronic renal diseases results in excess calcium and phosphorus deposition (calcification) in the blood vessels, thus hardening the tube wall, narrowing lumen, increasing blood pressure and aggravating tissue and organ ischemia, finally significantly increasing the incidence and mortality of cardiovascular events in patients. In the article of “Poly (ADP-ribose) polymerase 1 accelerates vascular calcification by upregulating Runx2”, Dr Wang Cheng and Dr Xu Wenjing in the team found that the abnormal activation of type I poly ADP-ribose polymerase (PARP1) in vascular smooth muscle cells was an important factor for vascular calcification in chronic kidney diseases (CKD):activated PARP1 resulted in the loss of normal contractile function of smooth muscle cells and the transformation into osteoblasts/chondrocyte-like cells, expressing calcified proteins and secreting bone-related matrix, and contributing to the calcification of blood vessels. Further studies revealed that PARP1 reduced mir-204 expression by promoting the expression of inflammatory factor IL-6 and activating the JAK2/STAT3 pathway, thereby eliminating theinhibition of mir-204 on the transcription factor Runx2, leading to the increased expression of ossification related proteins (Col1A1, OPN, OCN) and promoting calcification. From the introduction of the corresponding author Professor Huang Kai, the head of metabolic cardiovascular disease research team of Union Hospital, , this study revealed that PARP1 played the key role in the process of vascular calcification in patients with chronic kidney diseases, and calcification was not the passive deposition of calcium and phosphorus in blood vessel walls, but an active and controllable biological process, which thus provided a new idea and therapeutic target to solve the occurrence, development and progression of kidney disease in patients with vascular calcification.
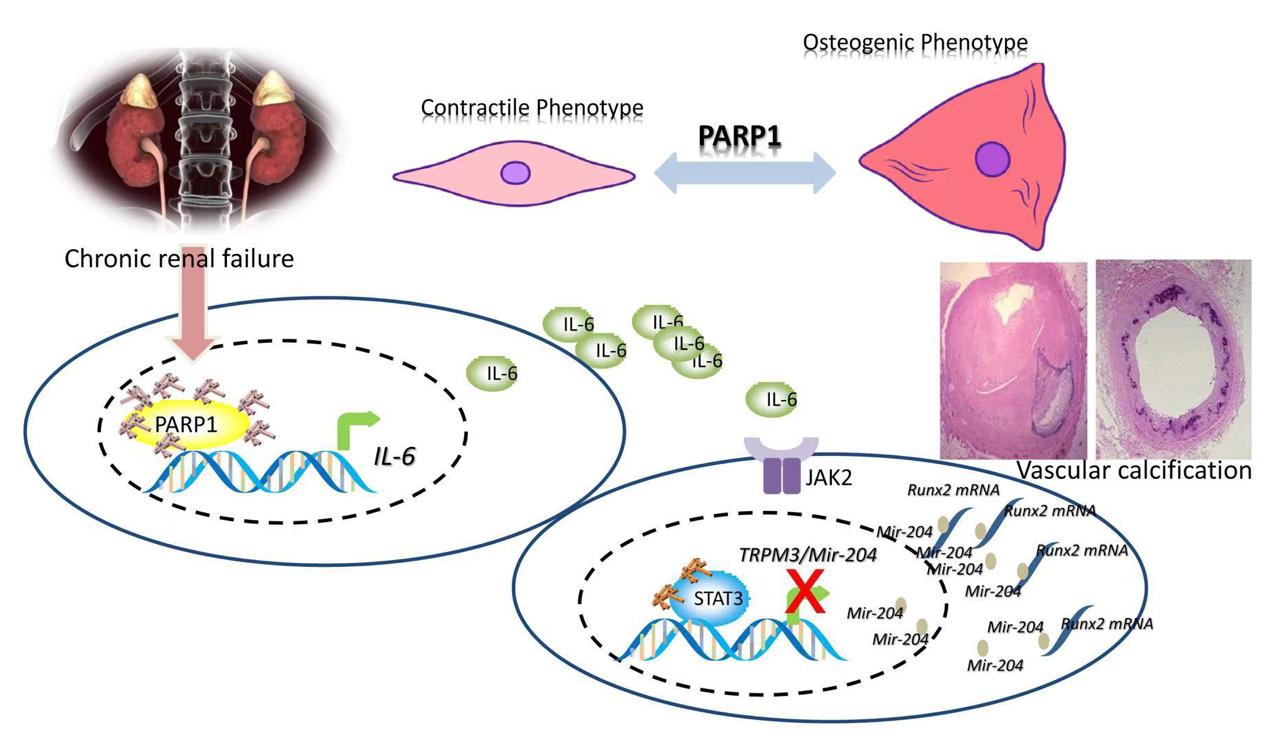
Figure 1 PARP1 regulated the vascular calcification
The cooperative research "Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells" published in Nature Communications on February 28, was mainly undertaken by the team. Associate professor, PI Cheng Min of the team was the first author and the main collaborator of the team, Professor Qin Gangjian of University of Alabama at Birmingham, the distinguished professor of our university, was the corresponding author. The authors found that the myocardial specific microRNA (myo-miR:miR-1a, miR-208a, miR-133a and miR-499-5p) carried by exosomes in peripheral blood of patients with acute myocardial infarction was significantly increased, up to 10,000 to 100,000 times than normal patients. Animal experiments further indicated that these myo-miRs were transferred into the bone marrow(BM) by exosomes, and promoted the release of BM progenitor cells into the blood by inhibiting the expression of CXCR4 gene in BM progenitors cells, which could medicate the repairment of damaged myocardium. Professor Cheng Min said that this study revealed that peripheral blood myo-miRs were not only a biomarker, but also an important molecule mediating the signal transmission between ischemic heart and BM. In view of the important role of BM progenitor cells in myocardial repair, the discovery of this new intra-organ signaling pathway was expected to provide a new target for the treatment of ischemic heart disease. Professor Huang Kai, who is the director of the metabolic cardiovascular disease research center in Union hospital, said that the high mortality rate of ischemic heart disease was a severe health issue in word wide. This research achievement would provide an important target for the treatment of myocardial infarction, especially for stem cells medicated cardiovascular repair.
