On January 25, Prof. YAO Guangmin 's team from School of Pharmacy and Prof. FANG Chao 's team from School of Basic Medicine published a research paper entitled《Piericones A and B as Potent Antithrombotics : Nanomolar Noncompetitive Protein Disulfide Isomerase Inhibitors with an Unexpected Chemical Architecture》on theJournal of the American Chemical Society( IF : 16.383 ).

Thrombotic-related diseases such as ischemic stroke and myocardial infarction are common cardiovascular and cerebrovascular disorders that seriously endanger human health with a high morbidity and fatality rate. Platelet activation and aggregation are critical steps during thrombus formation; however, antiplatelet agents such as aspirin and clopidogrel are commonly associated with increased bleeding risk and/or treatment failure to various degrees.
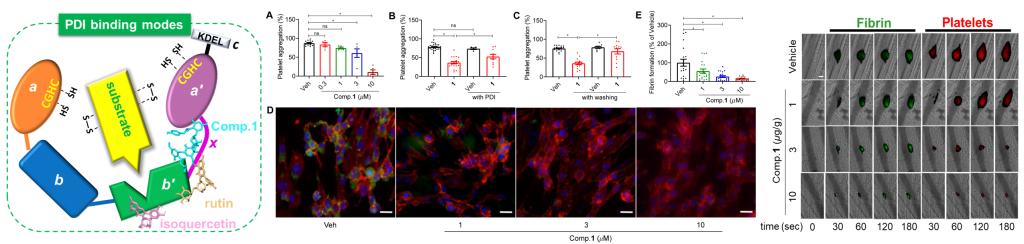
Protein disulfide isomerase (PDI), the prototypic member of the thiol isomerase family, catalyzes the oxidation, reduction, and isomerization of protein disulfide bonds. It contains four thioredoxin-like domains (a, b, b′, and a′), a linker region (x) between b′ and a′, and a C-terminal extension (c) right after the a′ domain. The substrate binding site of PDI is located in the b′ domain. Extracellular PDI plays a critical role in thrombosis by regulating platelet aggregation and fibrin generation. Inhibition of extracellular PDI is a promising strategy to develop novel antithrombotics without bleeding risks. Currently, diverse types of PDI inhibitors including antibodies, peptides, and synthetic small molecules have been reported. Among these, many peptide-based inhibitors irreversibly target the catalytic a and a′ domains with covalent bonds, resulting in the inactivation of PDI. Isoquercetin, a natural PDI inhibitor that binds to the b′ domain, had been reported to effectively reduce thrombosis in a Phase-II clinical trial. However, the low efficacy and bioavailability of quercetin derivatives limit their clinical application. Thus, it is of significant importance to find novel PDI inhibitors with antithrombotic potential.
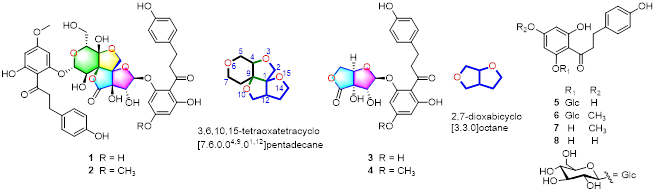
Four potent PDI inhibitors with unprecedented chemical architectures, piericones A–D (1–4), were isolated from Pieris japonica. Their structures were elucidated by spectroscopic data analysis, chemical methods, quantum 13C nuclear magnetic resonance DP4+ and electronic circular dichroism calculations, and single-crystal X-ray diffraction analysis. Piericones A (1) and B (2) were nanomolar noncompetitive PDI inhibitors possessing an unprecedented 3,6,10,15-tetraoxatetracyclo[7.6.0.04,9.01,12]pentadecane motif with nine contiguous stereogenic centers. Their biosynthetic pathways were proposed to include a key intermolecular aldol reaction and an intramolecular 1,2-migration reaction. Piericone A (1) significantly inhibited in vitro platelet aggregation and fibrin formation and in vivo thrombus formation via the inhibition of extracellular PDI without increasing the bleeding risk. The molecular docking and dynamics simulation of 1 and 2 provided a novel structure basis to develop PDI inhibitors as potent antithrombotics.
Prof.YAO Guangmin 's team, Dr. ZHENG Guijuan, is the first author of this paper, and Prof. YAO Guangmin and Prof. FANG Chao are the co-authors.
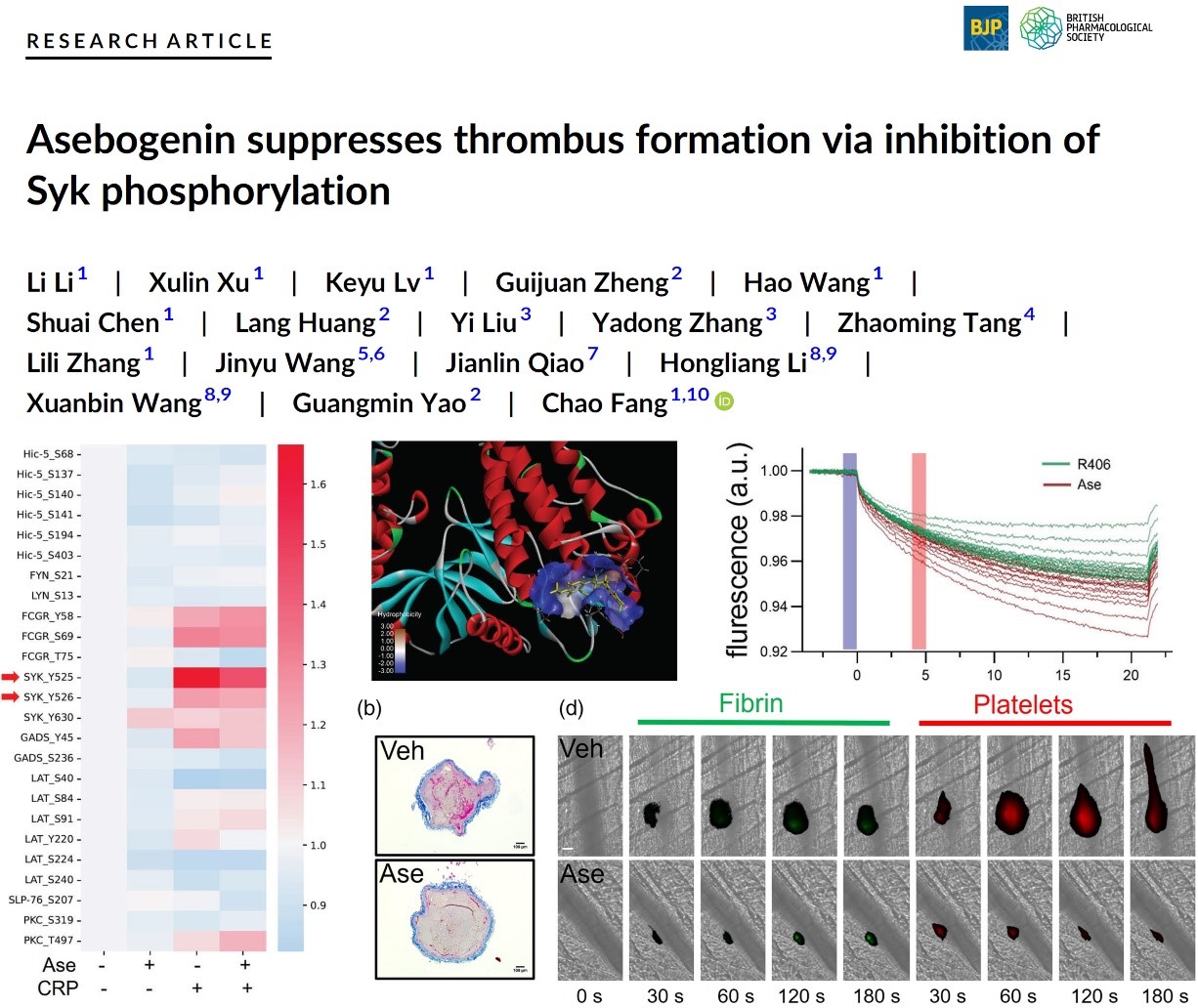
In-depth mechanistic studies have found that compound 7 attenuates Syk phosphorylation and related downstream signaling protein activation by targeting spleen tyrosine kinase ( Syk ), and reduces thrombosis by inhibiting platelet aggregation and fibrin production, and inhibits venous thrombosis.
The results of this part of the study were published in theBritish Journal of Pharmacology(2023, 180, 287−307, IF 9.473 ) with the title of ' Asebogenin inducing thrombus formation via inhibition of Syk phosphorylation '.
Dr. LI Li and Associate Professor XU Xulin of Prof. FANG Chao 's team are the co-first authors of this paper, and Prof. FANG Chao and Prof. YAO Guangmin are the co-communicating authors.
The relevant research results have been applied for three Chinese invention patents ( application numbers are 202211294113.8, 202210087140.1 and 202211141485.7, respectively ).
The above research work was supported by the National Natural Science Foundation of China, the National Science and Technology Research Program, the National Key R & D Program of the Ministry of Science and Technology, the Hubei Outstanding Youth Fund and the China Postdoctoral Fund.
Paper linnk:
https://doi.org/10.1021/jacs.2c12963
https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15964
