August 8, 2023. Nature Communications online published the latest research results of Professor WANG Weimin 's team in the Department of Immunology, Tongji Medical College, Huazhong University of Science and Technology, entitled " Intermittent dietary methionine deprivation promotes tumor ferroptosis and synergizes with checkpoint blockade. " Studies have found that long-term deficiency and transient starvation of methionine have opposite regulatory effects on ferroptosis of tumor cells. In vivo, intermittent methionine deprivation in the diet can significantly enhance the sensitivity of tumors to ferroptosis, and synergistically inhibit tumor progression with PD-1 antibody, enhancing T cell-mediated anti-tumor immune response.

Methionine is a sulfur-containing essential amino acid. It is not only used for protein synthesis, but also plays an important role in regulating methylation reaction, sulfur-containing amino acid metabolism, maintaining redox homeostasis and signal transduction. Since methionine can only be obtained from food, and the growth of many tumor cells is highly dependent on exogenous methionine, dietary methionine restriction has become a promising cancer treatment strategy. A large number of previous studies have shown that methionine restricted diet can inhibit the growth of various types of tumors in mice and enhance the sensitivity of tumors to chemotherapy and radiotherapy. However, the effect of methionine diet intervention on targeted tumor ferroptosis therapy and immunotherapy is still unclear.
Ferroptosis is an iron-dependent regulatory cell death triggered by abnormal lipid peroxide accumulation. It has been found to be closely related to physiological and pathological processes such as tumors, neurodegenerative diseases, cardiovascular diseases and acute kidney injury. Professor WANG Weimin focused on the interaction between ferroptosis and tumor immunity. For the first time, he found that tumor-infiltrating CD8 + T cells could inhibit cystine metabolism in the microenvironment ( Cell, 2016 ) and enhance the sensitivity of tumor cells to ferroptosis ( Nature, 2019 ). At the same time, he proposed a new strategy of ferroptosis inducers synergistically enhancing the efficacy of PD-1 antibody ( Cancer Cell, 2021 ). Therefore, it is of great significance to find new ferroptosis inducers or sensitizers to target ferroptosis of tumor cells for the treatment of tumors alone and combined immunotherapy.
In most cells, the cysteine-GSH-GPX4 axis is the most important metabolic pathway to protect cells from ferroptosis. Most of the intracellular cysteine is derived from the extracellular uptake of cystine, but a small part of it can be generated through the transsulfuration pathway of methionine. Studies have shown that methionine deprivation or inhibition of the transsulfuration pathway can promote ferroptosis in some tumor cells. In the previous work, WANG Weimin 's team found that the lack of cystine or methionine in the culture medium alone could reduce the GSH content in tumor cells.Surprisingly, cystine deficiency alone can significantly induce ferroptosis in a variety of tumor cells, but the simultaneous lack of methionine does not promote ferroptosis, but inhibits ferroptosis induced by cystine deficiency. Similarly, methionine deficiency also significantly inhibited erastin-induced ferroptosis. After detecting the GSH content in the cells, it was found that the lack of methionine reversed the decrease of GSH caused by cystine deficiency. More interestingly, when the tumor cells were subjected to a short ( £ 8h ) methionine starvation pretreatment followed by cystine deficiency alone, the ferroptosis of tumor cells was promoted, and the intracellular GSH content decreased. In summary, the long-term deficiency and short-term starvation of methionine have opposite regulatory effects on ferroptosis of tumor cells.
At the same time, the author has conducted in-depth research on the molecular mechanism behind the above phenomena. In the process of cystine deprivation or erastin-induced ferroptosis, de novo synthesis of CHAC1 protein is accompanied, which can enhance the degradation of GSH and promote the occurrence of ferroptosis. However, the long-term deficiency of methionine inhibits the protein synthesis of CHAC1, thereby inhibiting the occurrence of ferroptosis. Unlike long-term deficiency, transient methionine starvation only promotes the transcription of CHAC1 without inhibiting its protein translation, thus enhancing GSH degradation and ferroptosis.
Based on this, the authors propose that intermittent methionine diet deficiency is conducive to the occurrence of ferroptosis in tumor cells in vivo. The experimental results showed that the lack of intermittent methionine diet could significantly enhance the anti-tumor activity of the ferroptosis inducer IKE, and the diet alone could inhibit tumor growth. Furthermore, the authors also explored the combined therapeutic effect of intermittent methionine diet deficiency and PD-1 antibody in vivo. The results showed that the combination of the two could significantly inhibit tumor growth and enhance T cell immune response, and the effect of combined treatment was significantly better than that of single treatment. The therapeutic effect depends on the expression of CHAC1 in tumor cells. In the specimens of melanoma patients who underwent immunotherapy, the authors found that the high expression of CHAC1 was positively correlated with treatment responsiveness and patient survival. Finally, the authors found that the triple combination therapy of intermittent methionine diet deficiency, ferroptosis inducer IKE and PD-1 antibody could further inhibit tumor progression and prolong the survival time of tumor-bearing mice.
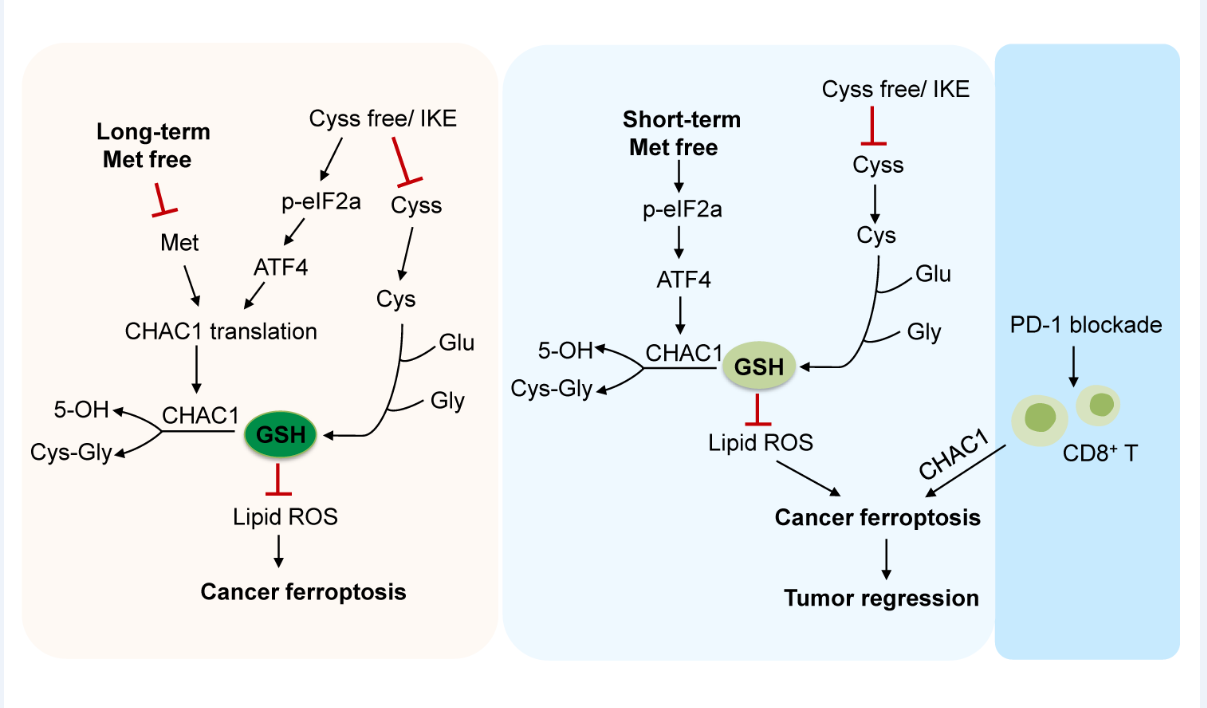
The different regulatory effects of long-term methionine deficiency
and transient starvation on ferroptosis
In summary, this study further revealed the dual role of methionine in the regulation of ferroptosis, clarified the interaction between methionine metabolism, tumor ferroptosis and anti-tumor immune response, and innovated people 's understanding and cognition of the regulation of tissue cell death and immune response by special diet under physiological conditions in vivo, which laid a theoretical foundation for the clinical transformation of diet intervention in amino acid metabolism for tumor treatment.
Professor WANG Weimin, Department of Immunology, Tongji Medical College, Huazhong University of Science and Technology, is the corresponding author of this paper. XUE Ying and LU Fujia, Ph.D.graduates of Department of Immunology, are the co-first authors of this paper. The research work is supported by the National Natural Science Foundation of China Youth and General Project, the Key Research and Development Program of the Ministry of Science and Technology, the Outstanding Youth Project of Hubei Provincial Natural Science Foundation and the Independent Innovation Fund of Huazhong University of Science and Technology.
Paper link:https://www.nature.com/articles/s41467-023-40518-0
