A research team led by Professor XIANG Wenpei from the Reproductive Health Research Institute at Tongji Medical College, HUST, has uncovered a novel mechanism that regulates the aging of bone marrow mesenchymal stem cells (BMSCs). This discovery, appearing in the journal Aging Cell, sheds light on the role of miR-203-3p in promoting BMSC senescence and its implications for age-related osteoporosis.

Stem cell aging is a critical event in the aging process of tissues and organs, contributing to the decline in their functionality and repair capabilities. BMSC aging, in particular, plays a significant role in the development of degenerative skeletal diseases. Despite its importance, the underlying causes of BMSC aging have not been fully understood.
In their study titled “miR-203-3p promotes senescence of mouse bone marrow mesenchymal stem cells via downregulation of Pbk,” the researchers conducted a comprehensive transcriptome sequencing and in vitro and in vivo experiments on BMSCs from young (3-week-old) and aged (60-week-old) mice. They demonstrated that high expression of miR-203-3p in aged BMSCs can regulate cellular functions through the Pbk/p53 signaling pathway, thereby affecting the bone marrow cavity microenvironment and promoting the occurrence of age-related osteoporosis.
Their initial analysis of the transcriptome data from young and aged BMSCs, combined with miRNA chip data from mice with median lifespans, identified miR-203-3p as a key gene in BMSC aging. Overexpression and silencing of miR-203-3p in these cells revealed that its high expression leads to reduced cell growth and increased senescence, while its downregulation can partially rescue the senescent phenotype.
Further investigation into the mechanism by which miR-203-3p regulates BMSC aging involved multi-site target gene prediction and dual-luciferase reporter assays. These experiments confirmed that miR-203-3p can directly bind to Pbk mRNA and negatively regulate its transcription. The researchers also found that Pbk can effectively rescue the senescence induced by miR-203-3p overexpression. As a member of the MAPKK protein family, Pbk is known to regulate p53 activity, either directly or indirectly. The team discovered an interaction between miR-203-3p and Pbk, and that suppression of Pbk by miR-203-3p can prevent ubiquitin-mediated p53 degradation, thus promoting BMSC senescence.
In a significant finding, the team administered an adeno-associated virus containing miR-203-3p inhibitor to the bone marrow cavity of aged mice. The treatment led to increased Pbk expression and decreased p53 expression in the bone marrow cavity. Most importantly, the treatment significantly increased bone volume fraction, trabecular thickness, and trabecular number in the mice's femurs, suggesting that inhibition of miR-203-3p in the bone marrow cavity can delay the loss of osteoblasts, which might potentially serve as a target for developing strategies to improve age-related osteoporosis.
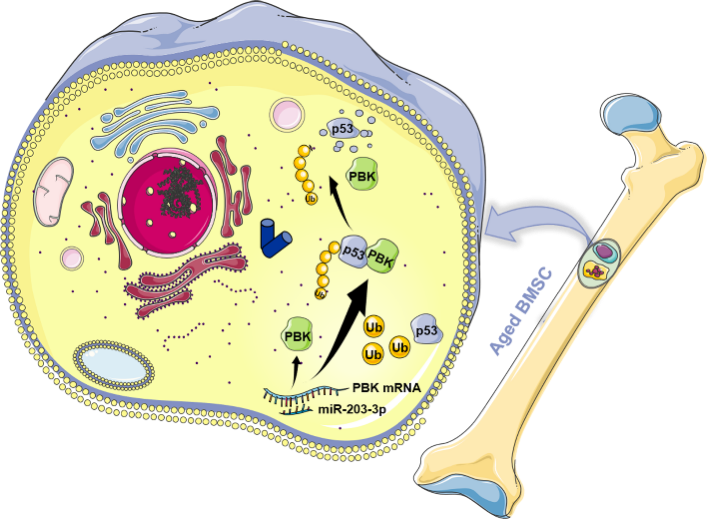
Conclusion and Future Prospects: This study, for the first time, revealed the significant role of miR-203-3p in BMSC aging and elucidated its molecular mechanism. It innovatively suggests that miR-203-3p might play a role in delaying age-related osteoporosis, thereby providing a new target for the interventions for degenerative skeletal diseases.
