A groundbreaking study by the team of Professors WU Tangchun and WANG Chaolong, from HUST, in collaboration with Professor LV Yun from Peking University, has unveiled novel DNA methylation signatures associated with the development of Acute Coronary Syndrome (ACS). The research, published in the prestigious journal Nature Communications, provides significant insights into the epigenetic mechanisms that may contribute to the onset of ACS, a severe form of coronary heart disease.
ACS, which encompasses acute myocardial infarction and unstable angina, is a leading cause of mortality worldwide. Despite advancements in prevention, diagnosis, and treatment, the condition continues to pose a significant health risk. Their study focused on DNA methylation, a reversible epigenetic modification, and offered a dynamic perspective to apporach the risk beyond genetic predispositions.
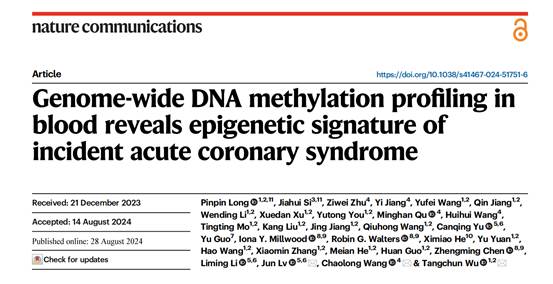
The research consisted of a two-stage Epigenome-Wide Association Study (EWAS), conducted across two large-scale Chinese prospective cohorts: the Dongfeng-Tongji (DFTJ) cohort and the China Kadoorie Biobank (CKB) cohort. The first phase identified 72 differentially-methylated positions (DMPs) associated with ACS episodes from over 770,000 methylation sites in 751 case-control pairs from the DFTJ cohort. The second phase confirmed 26 DMPs with consistent and significant associations in 476 case-control pairs from the CKB cohort.
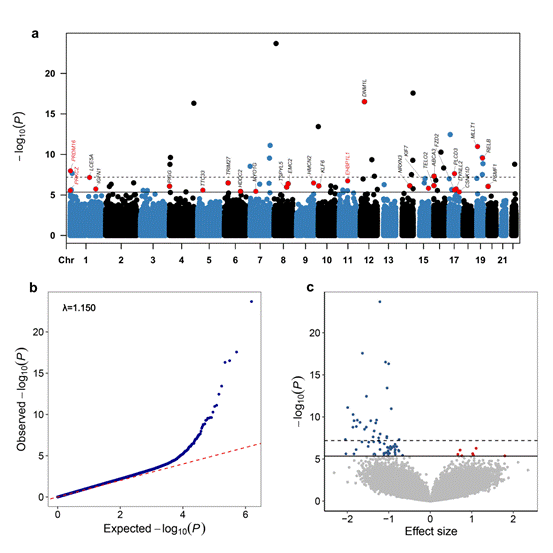
Notably, the study employed Mendelian Randomization analysis to establish a causal link between four DMPs located in the genes PRKCZ, TRIM27, EMC2, and EHBP1L1, and the risk of ACS. Furthermore, the research team conducted an expression quantitative trait methylation analysis (eQTM) to assess the regulatory potential of these DMPs on gene expression, revealing significant negative correlations between methylation levels and gene expression in certain ACS-related genes.
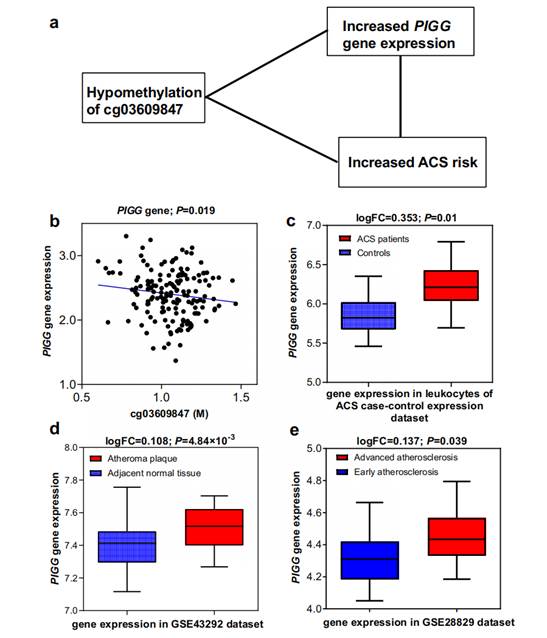
The most promising finding of the study is the development of a methylation risk scoring (MRS) scale based on 67 DMPs, which outperformed traditional risk factors and polygenic risk scores (PRS) in predicting ACS risk across both cohorts. This highlights the potential of DNA methylation as a biomarker for early disease prediction and intervention.

"The identification of these DNA methylation signatures not only enhances our understanding of the pathogenesis of ACS but also opens up new avenues for the development of personalized preventive and therapeutic strategies," said Professor WU Tangchun.
The research team is optimistic about the future implications of their findings and is planning to validate these results in diverse populations and animal models to further explore the underlying biological mechanisms. This could lead to more targeted approaches in precision medicine and early intervention strategies for ACS.
