On 29 October 2024, Prof. XIONG Bo's team in the Department of Forensic Medicine, Tongji Medical College, Huazhong University of Science and Technology (HUST) published a paper entitled "Deficiency of DDX3X results in neurogenesis defects and abnormal behaviours via dysfunction of the DDX3X syndrome" in the prestigious international journal Proceedings of the National Academy of the Sciences of the United States of America (PNAS) online. The study examined the human genetic profile of de novo mutations in DDX3X and revealed the pathogenic mechanism by which DDX3X leads to abnormal mental behaviours via the regulation of neuronal cell fate. This result provided a potential pathway to alleviating the core symptoms of this neurodevelopmental disorder.

Neurodevelopmental disorders refer to a group of mental conditions of children characterized by cognitive, emotional, and behavioural abnormalities during development, resulting from genetic, environmental, and other reasons, and mainly include autism spectrum disorders, intellectual disabilities, developmental delays, and attention deficit hyperactivity disorder (ADHD), among others. This group of disorders seriously affects the ability of the affected children to live independently and poses a serious social burden. Currently, hundreds of risk genes have been found to be potentially implicated in the development of neurodevelopmental disorders, with high genetic heterogeneity. Understanding of its underlying pathogenic mechanisms can help identify potential therapeutic targets and provide a basis for more precise treatment of their core symptoms.
The study first looked into the whole-exome or whole-genome sequence data from 46,612 families of patients primarily diagnosed as having autism or developmental delay, collected through international collaborations, and confirmed that DDX3X was the second most common de novo mutation risk gene for neurodevelopmental disorders, which contributed to the risk of developmental delays predominantly in female patients and was evolutionarily-conserved.
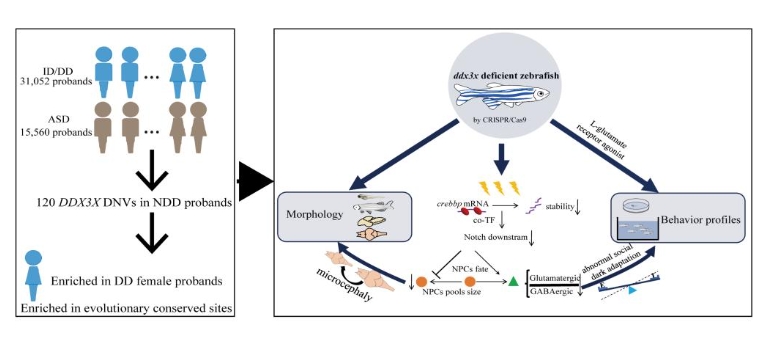
To investigate the role of DDX3X in neurodevelopment, XIONG's team constructed zebrafish models with different doses of deletion using CRISPR-Cas9 gene editing technology. In the animal model, they found that DDX3X-deficient zebrafish morphologically had reduced survival, developmental delay, microcephaly, and lowered brain connectivity; and behaviorally, adaptive deficits, social deficits, and spatial memory deficits. Further cytological studies revealed that DDX3X deficiency resulted in a reduced pool of neural stem cells, a decrease in the total number of neurons, and an imbalance in excitatory and inhibitory neuronal differentiation. On the other hand, supplementation with glutamate or glutamate agonists alleviated the adaptive and social deficits. Mechanistically, this study revealed that DDX3X deficiency ultimately led to abnormal neuronal cell fates by decreasing the RNA stability of its bound CREBBP, which, in turn, inhibited NOTCH signalling, and genetic or pharmacological activation of CREBBP or NOTCH signaling ameliorated the abnormalities of such neuronal cell fates. This study clarified the relationship between DDX3X mutations and neurodevelopmental disorders, and analysed the potential cytological and molecular mechanisms by which DDX3X leads to neurodevelopmental disorders, which might inform the development of possible therapeutic strategies.
Professor XIONG was the corresponding author of the paper, and Wei Duan, a postdoctoral fellow of the Department of Forensic Medicine, was the lead author. This study was supported by the National Natural Science Foundation of China under the Innovative Group , Top Project, Youth Project , and the Technology Innovation Fund of Innovation Research Institute of Huazhong University of Science and Technology .
