MFN1 (Mitofusin 1), a key regulatory protein for mitochondrial outer membrane fusion, has been proven to play a significant role in the differentiation of spermatogonia in male mice and follicular development in female mice. However, the specific function of MFN1 in preimplantation embryonic development and its potential association with epigenetic reprogramming have not been fully elucidated. This study aims to investigate the function and potential mechanisms of MFN1 in preimplantation embryonic development in mice.
On March 17, 2025, Professor. Liquan Zhou, Professor. Ximiao He, and Associate Professor. Bingxin Ma from Tongji Medical College of Huazhong University of Science and Technology published a research paper titled "Mitofusin 1 Drives Preimplantation Development by Enhancing Chromatin Incorporation of Histone H3.3" in the journal Advanced Science. The study, employing microinjection techniques combined with IP-MS, Co-IP, low-input RNA sequencing, and ChIP-seq, elucidated the novel regulatory mechanisms of MFN1 in early mouse embryonic development. This study reveals that MFN1 not only participates in mitochondrial dynamics but also forms complexes with cytoplasmic lattice proteins, and regulates epigenetic reprogramming during early embryonic development by enhancing expression of histone variant H3.3.
In this study, the authors firstly identified the continuous expression of MFN1 during oocyte maturation and early embryonic development through analysis of immunofluorescence staining results, RNA-seq dataset and Ribo-seq dataset from mouse oocytes and early embryos. MFN1 deficiency led to a series of developmental abnormalities, as shown below: MFN1 depletion in GV oocytes impaired oocyte maturation; MFN1 depletion during meiosis disrupted male pronuclear formation; MFN1 depletion in MII oocytes decreased zygotic genome activation (ZGA) activity, resulting in subsequent embryonic arrest at the two-cell stage; MFN1 depletion in zygotes led to embryonic development arrest at the morula stage. These results demonstrate the crucial function of MFN1 in early mouse embryonic development.
To further explore the mechanisms by which MFN1 regulates pronuclear formation and early embryonic development, the authors used IP-MS to identify an interaction between MFN1 and the cytoplasmic lattice component PADI6. Immunostaining experiments after cell expansion also showed strong colocalization of MFN1 and PADI6 in oocytes. Combined with H3.3 knockdown experiments, the authors confirmed that MFN1 deficiency disrupts the cytoplasmic lattice structure and ribosomal protein subunit assembly, leading to decreased expression levels of translation-sensitive histone H3.3 and impaired male pronuclear formation.
Subsequently, H3.3 knockdown assay with low-input RNA sequencing and ChIP-seq analysis revealed significant reduction in H3.3 enrichment at chromatin in the absence of MFN1. Analysis of chromatin distribution showed that H3.3 localization on the genome is positively correlated with the enrichment levels of RNA polymerase II (Pol II) and active histone modification H3K27ac. In contrast, the distribution of Pol II and H3K27ac on the embryonic genome was markedly weakened following MFN1 and H3.3 deficiency. These results indicate that MFN1 promotes early embryonic gene transcription activity by mediating the deposition of H3.3 at the transcription start sites of actively transcribed genes.
Finally, the authors discovered that supplementation with the small molecule MFN1 agonist S89 significantly improved mitochondrial function and increased H3.3 protein expression levels in early embryos from maternally aged female mice, thereby promoting embryonic development. Additionally, the study found that in human early embryos, MFN1 protein expression levels are positively correlated with H3.3 protein levels, both of which significantly decline with maternal age. This provides new insights into age-related early embryonic developmental defects and holds potential clinical value in reproductive medicine.
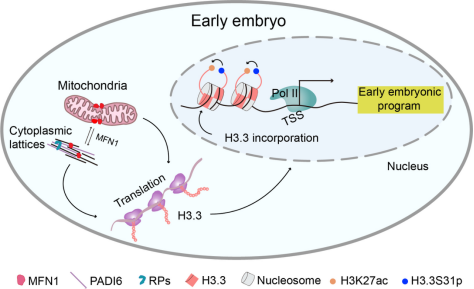
In summary, this study firstly proposes that MFN1 regulates mitochondrial and cytoplasmic lattice functions in early mouse embryonic development, promotes the incorporation of histone H3.3 into embryonic chromatin, ensures normal pronuclear formation and successful development of 2-cell embryos, and maintains precise control of early embryonic developmental programs. The study elucidated the non-canonical mitochondrial fusion function of MFN1 in preimplantation embryonic development and its involvement in epigenetic reprogramming, providing new perspectives for clinical research on maternal aging-related human embryonic developmental defects potentially mediated by MFN1 deficiency.
Professor Liquan Zhou from the Institute of Reproductive Health at Tongji Medical College of Huazhong University of Science and Technology, Professor Ximiao He from the School of Basic Medical Sciences, and Associate Professor Bingxin Ma from the Affiliated Tongji Hospital are the co-corresponding authors of this paper. Xiaoyan Shi, 2021 Doctor candidate from the Institute of Reproductive Health, is the first author. Doctor candidate Yu Tian, Master candidate Yufan Wang, and Doctor candidate Yiran Zhang also made significant contributions to this study. This work was supported by the National Natural Science Foundation of China and the Program for HUST Academic Frontier Youth Team. (Correspondent Shi Xiaoyan)
